Small molecule crystallography
The crystal samples studied are typically, inorganic, organic or organo-metallic compounds, primarily from research in the disciplines of chemistry, geology and physics.
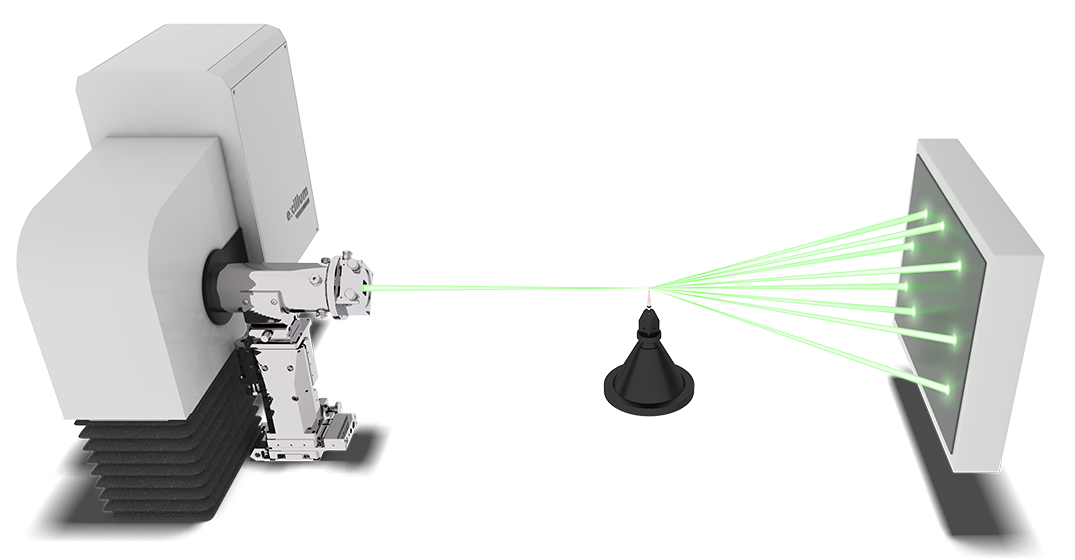
As small molecule X-ray crystallography becomes more automated and routine, there is increasing interest in the study of more difficult and specialist materials where the high brilliance Excillum MetalJet X-ray source is desirable. Use of the MetalJet typically means shorter experiment times, faster structures and higher throughput of samples. Small and weakly diffracting crystals, diffract more strongly providing higher quality data, whilst sensitive crystals can be measured faster with the MetalJet and suffer less degradation accordingly. Twinned crystal data can be more strongly defined using a MetalJet, making it more easily identified and potentially handled. Very weak diffraction effects, inherent to incommensurates, diffuse scattering samples, quasi-crystals and high pressure samples become stronger and may be more readily measured and investigated using the high brilliance X-rays of a MetalJet.
Application examples for Small molecule crystallography
Fast data collection for high throughput
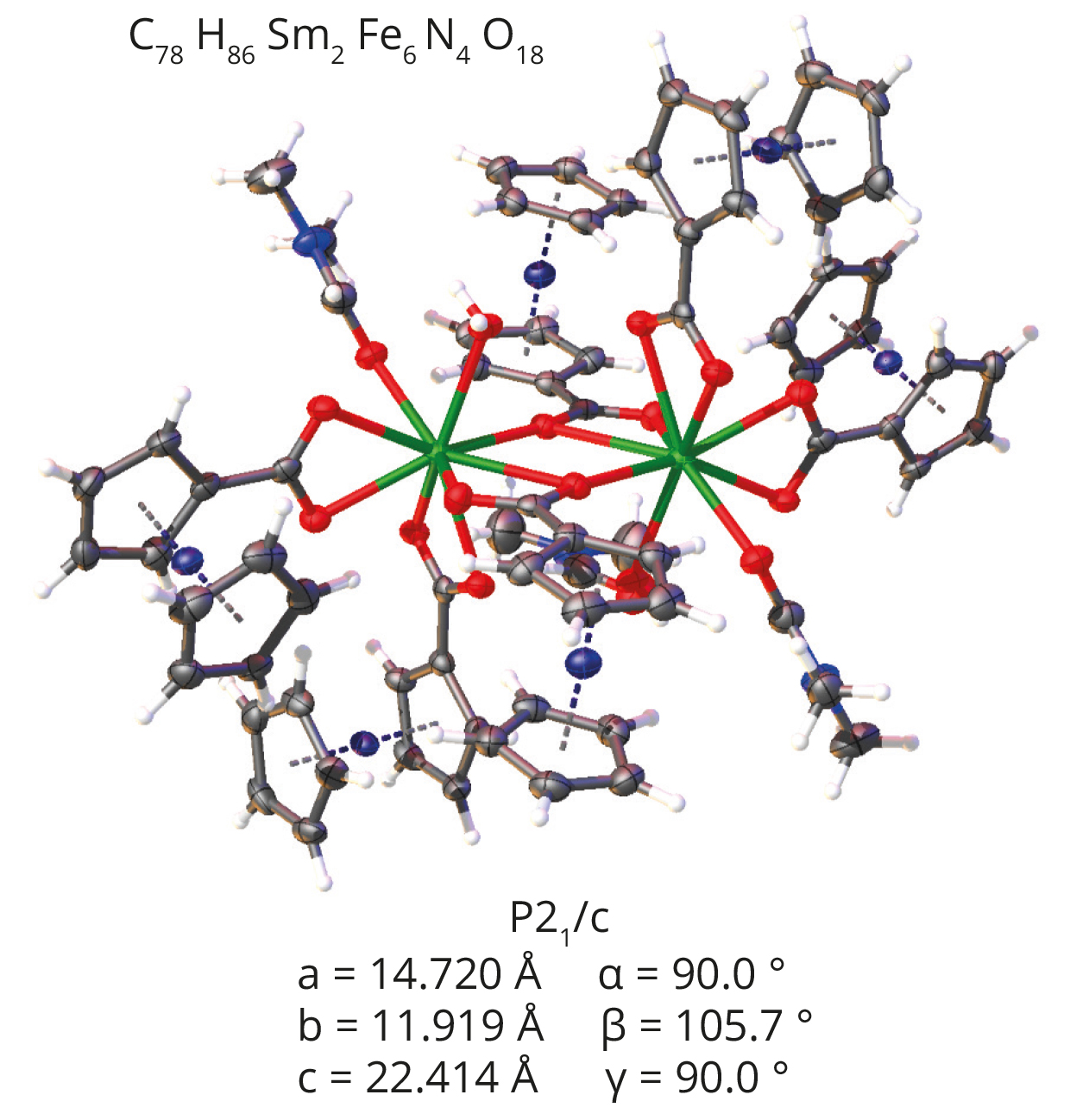
Scientist at STOE has compared the performance of a MetalJet setup with a standard microfocus tube. In this case they used the same ~100µm crystal (samarium ferrocene carboxylic complex) for both measurements.
- For the standard microfocus source, 1308 frames at 1 degree step was captured with 360 seconds exposure time. This led to a total measurement time of around 120 h yielding acceptable R1 = 4.43 % wR2 = 13.13 %.
- The MetalJet experiment was performed at 0.5 degree steps with 1 s exposure time resulting in a 40 min experiment. The corresponding reliability factors R1 = 2.64 % wR2 = 5.16 %.
Experiment performed at the STOE laboratory by Dr. rer nat Thomas Pippinger.
Small crystals
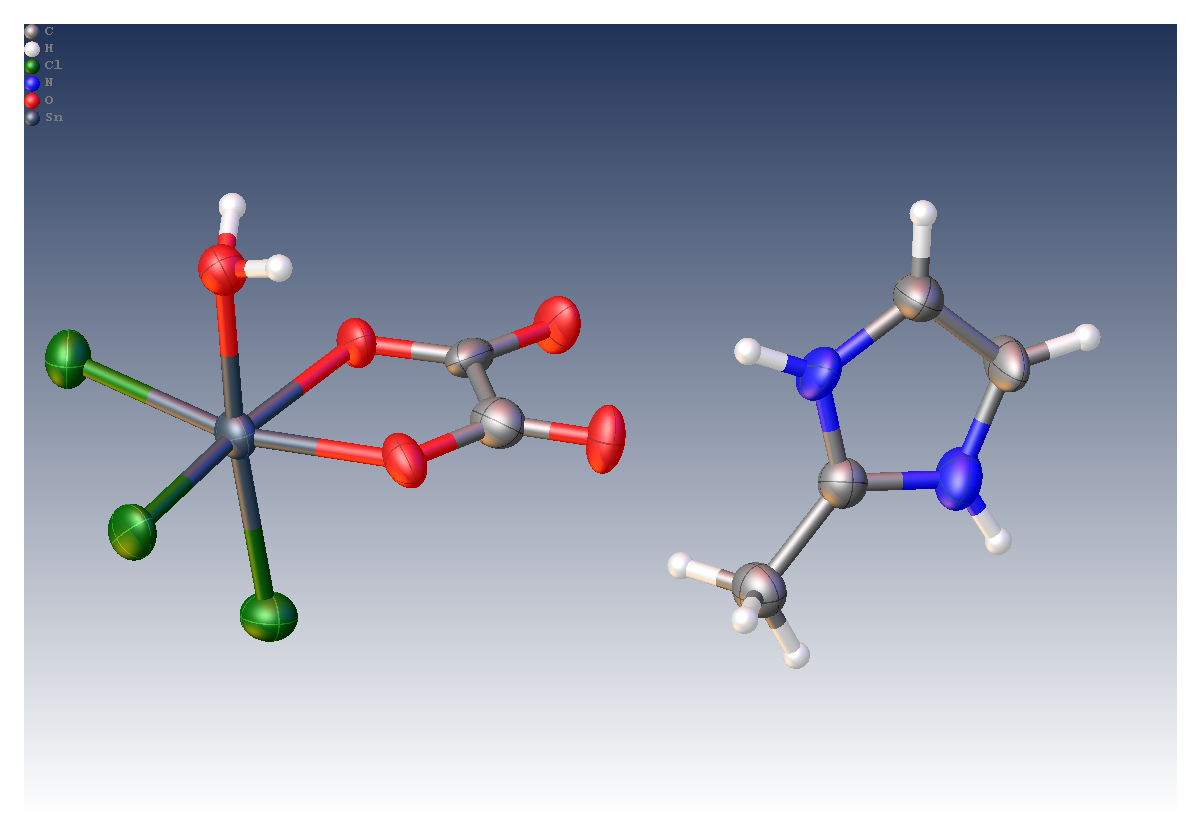
Tin (IV) compounds are interesting as potential catalysts and pharmaceuticals due to their biological activity. As part of a drive to understand these compounds, researchers at the Universities of Montreal, Cheikh Anta Diop and Bourgogne determined the crystal structure of a 50 μm crystal of [Sn(C2O4)Cl3(H2O)].(C4H7N2) using a MetalJet X-ray source.
- Crystal size: 0.05 x 0.04 x 0.04 mm3
- R1 = 6.2%
Researchers at the University of Hong Kong determined the crystal structure of a tiny crystal of C23H14F3N3ORh·CF3O3S using a Bruker Diffractometer with an Excillum MetalJet mounted, using Ga Kα, radiation (λ = 1.34138Å).
Crystal size: 0.04 × 0.01 × 0.01 mm3
Data collection time: 2 hours
R1 = 4.9 %
Completeness: 98.3%
Absolute structures
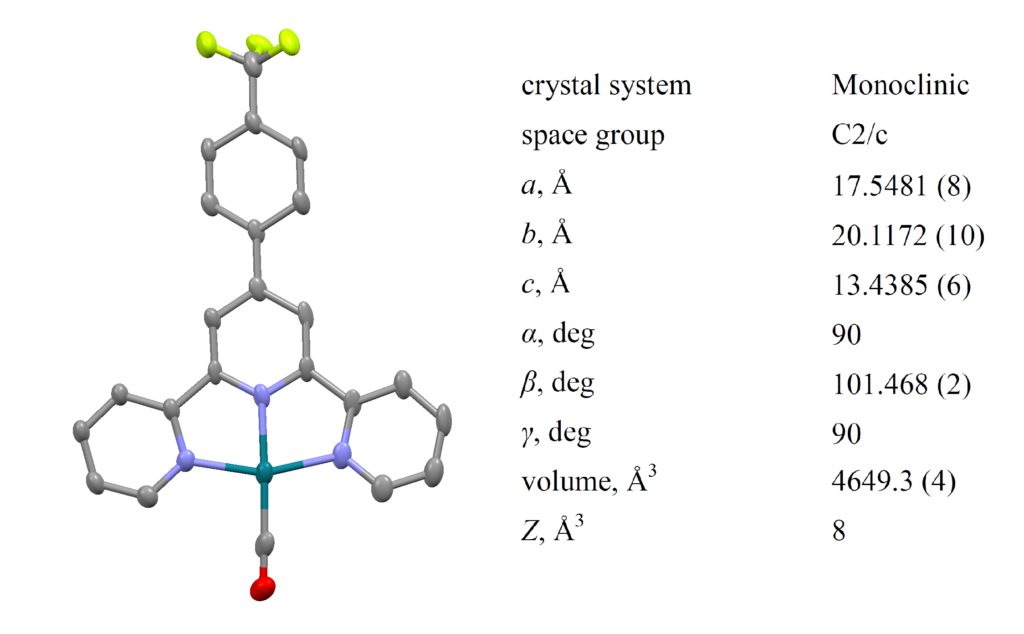
Application scientists at Bruker AXS used an Excillum MetalJet integrated into a Bruker D8 VENTURE diffractometer to successfully determine the absolute configuration of (2S)-(−)-2,2’-Oxybis(octahydro-7,8,8-trimethyl-4,7-methanobenzofuran), a light atom structure, where the heaviest atoms are three oxygen atoms.
- Flack x = 0.024(39) (Parsons‘)
- Crystal size: 0.15 x 0.05 x 0.04 mm3
- Experiment time: 4 hours
- Resolution: 0.75 Å
- Completeness: 96%
- Redundancy: 4
- R1 = 3.18%
Heavy absorbers
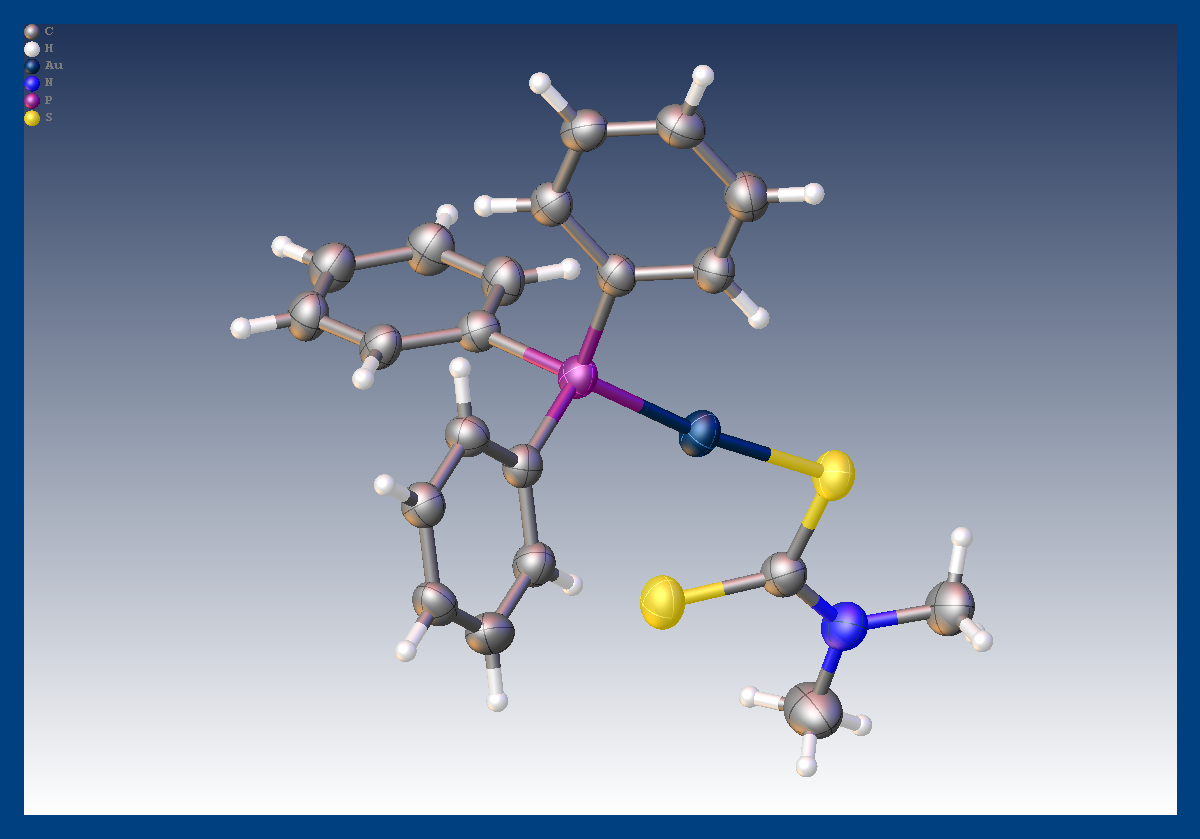
A group of researchers from the University of Montreal recently determined the crystal structure of the heavily absorbing compound [Au(PPh3) (S2CNMe2)] using an Excillum MetalJet source integrated into a Bruker D8 VENTURE. The compound was part of a study of materials based on d10 configured gold (I) compounds which have potential application in the area of luminescent sensors.
- Crystal size: 0.08 x 0.04 x 0.04 mm3
- R1 = 3.94
- Absorption coefficient μ (Ga Kα ) = 10.57 mm-1
Extended metal network structures
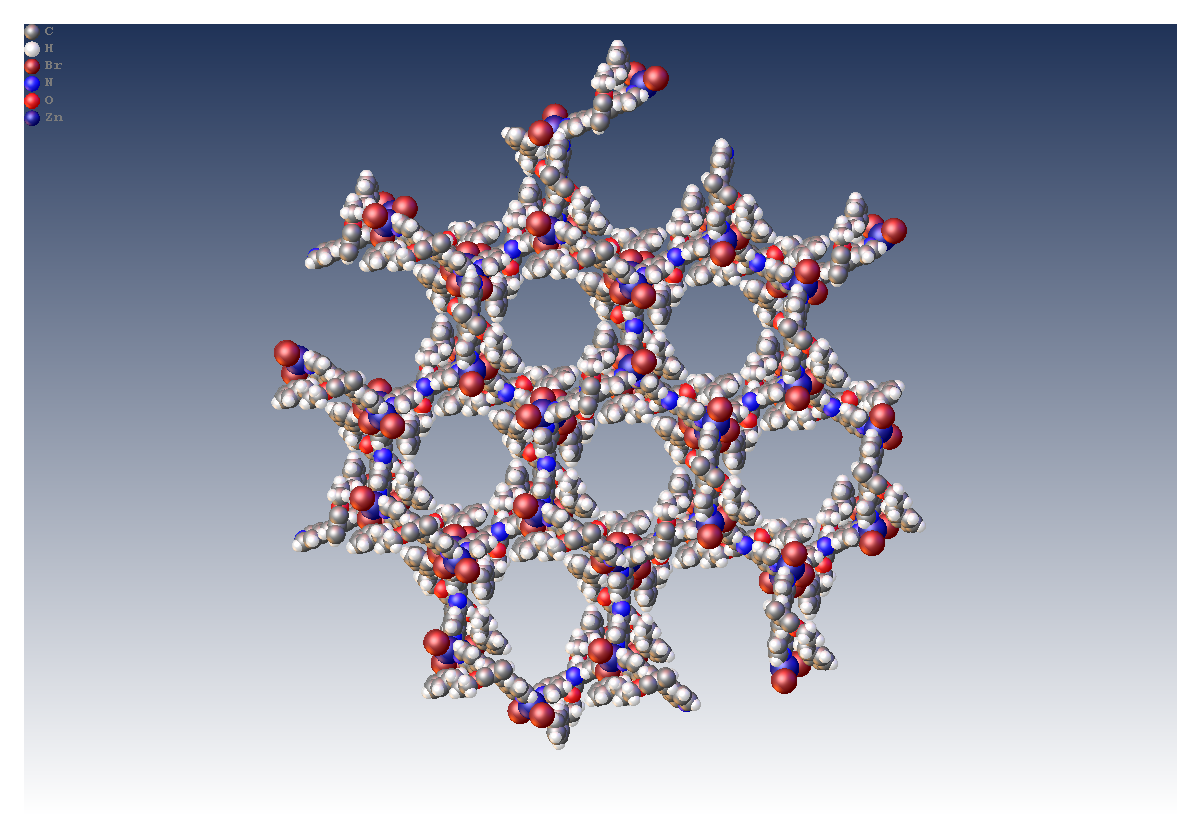
Researchers at the University of Basel have used an Excillum MetalJet mounted on a STOE diffractometer to study the crystal structures of a series of extended three dimensional metal network structures including [Zn2Br4(L)]n. This structure exhibited long unit cell edges and a large unit cell volume of 28133.2(9) Å3.
- a = 35.9593(6) Å
- b = 35.9593(6) Å
- c = 25.1227(3) Å
- R1= 8.98%